
Our First-in-Class Therapeutically Biological Drug JL15003 Approved for Clinical Trial by NMPA, CHINA
A Milestone for R&D, A New Start in 2022
On Dec 31st, our new drug named JL15003, an injection treatment for relapsed/refractory Glioblastoma has been approved by the Center for Drug Evaluation (CDE), NMPA, to conduct a clinical trial. This is a first-in-class new drug, as a “gift” to the company, it is also a new sailing for JECHO in the new year.
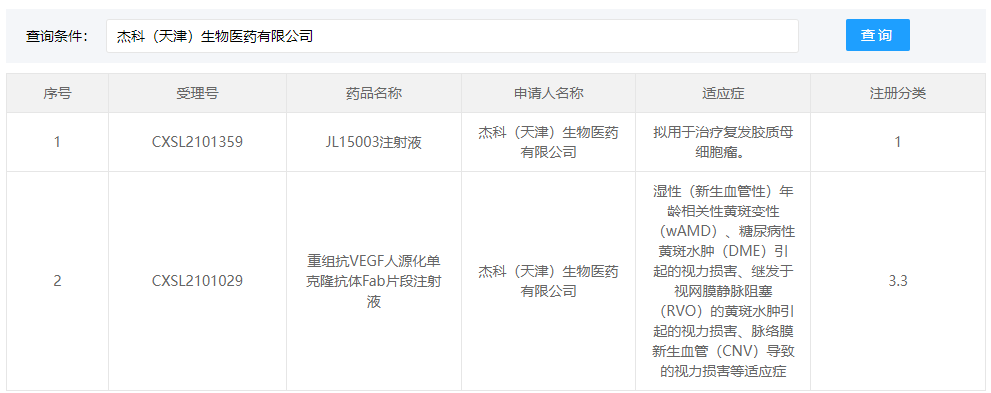
Source: Center for Drug Evaluation (CDE), NMPA, China
JL15003 injection aims to conquer relapsed/refractory Glioblastoma, and it was registered as Category 1. "This is another drug candidate in clinical trial stage after the JL14002 project which was approved to initiate Phase III clinical trials this year. It is an important milestone to JECHO new drug development. A series of in-depth research will be followed up." Dr. Zhu Jianwei, CEO and CSO of JECHO said.
In 2022, there will be more biopharmaceutical drugs stepping into the products pipeline. We expect the company’s innovation and development will serve regional biological industry next year.







